by Aesthetic Doctor, Dr Cindy Kerbel
In the realm of aesthetics, the intersection of pregnancy and cosmetic procedures presents a delicate balance between maternal well-being and the pursuit of beauty. The safety of cosmetic procedures for patients who are pregnant or breastfeeding is a topic that requires considerable thought and thorough discussion. Lack of data and evidence surrounding the safety of many aesthetic treatments often results in practitioners deferring treatment or paring back treatment plans until the pregnancy and lactating period is complete.
To better understand these concerns, let’s embark on a comprehensive exploration of the scientific landscape surrounding aesthetic injectables during pregnancy, delving into the risks, considerations, and recommendations for expectant mothers.
Table of contents
- AESTHETIC INJECTABLES: AN OVERVIEW
- TYPES OF AESTHETIC INJECTABLES
- DESIRED EFFECTS AND POPULARITY
- REGULATORY STATUS AND GENERAL SAFETY
- PREGNANCY: IMPLICATIONS FOR COSMETIC PROCEDURES
- HORMONAL CHANGES AND SKIN SENSITIVITY
- BLOOD VOLUME AND CIRCULATION ALTERATIONS
- IMMUNE SYSTEM ADJUSTMENTS
- THE RISK OF AESTHETIC INJECTABLES DURING BREASTFEEDING
- CONSIDERATIONS DURING PREGNANCY AND BREASTFEEDING
- FINAL THOUGHTS
AESTHETIC INJECTABLES: AN OVERVIEW
Aesthetic injectables encompass a diverse array of treatments aimed at enhancing facial appearance and addressing various skin concerns. Among the most commonly utilized injectables are neuromodulators, such as botulinum toxin, and dermal fillers, typically composed of hyaluronic acid. In recent years, the addition of complementary injectables for use in aesthetics, such as skin boosters, bio-remodelling agents and biostimulators, has broadened the scope of treatments and procedures that aesthetic practitioners can offer their patients.
TYPES OF AESTHETIC INJECTABLES
Neuromodulators
Neuromodulators, better known as botulinum toxin injections or neurotoxins, are primarily used to treat dynamic wrinkles – the visible lines on our faces formed by repetitive muscle contraction. By blocking the release of acetylcholine, a neurotransmitter responsible for muscle contractions, neuromodulators can temporarily relax facial muscles, diminishing the appearance of unwanted lines, and resulting in a smoother, more youthful and refreshed appearance. The effects of botulinum toxin injections last 3-4 months.
Dermal Fillers
Dermal fillers are primarily used in profile balancing and enhancing facial contours, smoothing out wrinkles, and restoring volume and facial support that has been lost as we age. The most commonly used fillers are hyaluronic acid-based dermal fillers. Hyaluronic acid is a naturally occurring substance in the skin that attracts and retains water (moisture), providing hydration and volumizing effects. Whether seeking a subtle enhancement or a more dramatic rejuvenation, hyaluronic acid fillers offer versatility and natural-looking results. The longevity of these treatments typically lasts 12-18 months.
Other injectables often used in aesthetics include biostimulators, bio remodelling agents and skin boosters.
Biostimulators
Biostimulators are biologically active substances that can be injected under the skin to improve the quality and functioning of the skin and substructures to firm and rejuvenate the skin.’ Two examples of biostimulators that are commonly used are calcium hydroxyapatite (CaHA) and Poly-L-Lactic acid (PLLA), both of which stimulate collagen formation. ‘CaHA particles support the body’s own collagen production by providing a structure for fibroblasts (collagen-producing cells) to rest on and form new collagen fibres. It is delivered in a carrier gel that slowly disappears over time. As this happens, a firm dense collagen network is built in its place for a gradual long-lasting lift. PLLA, on the other hand, recruits new fibroblasts to the treatment area, that stimulate the formation of additional collagen fibres, providing a more intense collagen stimulating effect.’ In both cases, visible lifting and tightening effects take time to develop, typically starting from 3 months onwards, with results lasting 1-2 years.
Bio Skin Remodellers
Bio remodelling refers to an injectable anti-ageing treatment that provides the combined benefits of a subtle lift from ordinary soft filler along with the skin quality-enhancing effects of a skin booster. This agent contains one of the highest concentrations of hyaluronic acid on the market, providing a significant hydration boost to the skin. In addition, it boosts the production of both collagen and elastin. Thus bio skin remodelling not only improves hydration but gives the skin a plumper, smoother, firmer appearance and diminishes the appearance of fine lines, with results lasting approximately 6 months.
Mesoboosters
Skin boosters are used to upregulate, rejuvenate, hydrate, and improve the quality and functioning of the skin. Comprising of a series of micro-injections, skin boosters can deliver vital nutrients, vitamins, and hyaluronic acid deep into the layers of the skin. This process aims to improve skin elasticity, reduce fine lines and provide a youthful glow.’ For optimal results (we recommend Mesoboosters) a series of treatments is often required. Results are gradual and are usually evident from 6 weeks-3 months post-treatment. Maintenance treatments are recommended for sustained results.
Stem Cells
Finally, stem cells can also be injected into specific areas of the face and neck (as with the StemCell4DLift). Stem cells are renowned for their regenerative prowess. As facial injectables, stem cells offer a revolutionary approach to enhancing volume and refining skin quality. Derived from adipose tissue, through the process of liposuction and fat grafting techniques, the fat is filtered into macro fat, micro fat and nano fat. These potent cells possess the remarkable ability to stimulate collagen production, promote tissue regeneration and restore youthful vitality to the skin. While stem cells can be considered as injectables in aesthetics, their use falls more accurately into the category of surgical procedures.
DESIRED EFFECTS AND POPULARITY
Pregnancy is a time of considerable physiological change and flux. Pregnant and breastfeeding women may express interest in aesthetic injectables to address hormonally driven skin changes such as hyperpigmentation and acne, as well as to enhance their self-esteem. Despite advisories against their use during pregnancy and breastfeeding, the allure of quick, non-surgical treatments remains appealing due to their perceived safety and minimal recovery time.
It is, however, crucial to emphasize the importance of professional medical advice before considering injectables during pregnancy and breastfeeding. While these treatments may seem relatively harmless, their safety profiles in these specific populations have not been thoroughly established, necessitating caution and informed decision-making.
REGULATORY STATUS AND GENERAL SAFETY
The regulatory landscape governing aesthetic injectables ensures rigorous evaluation of their safety and effectiveness before approval for clinical use. Entities like the Food and Drug Administration (FDA) and the South African Health Products Regulatory Authority (SAHPRA) play pivotal roles in assessing the risks and benefits of these treatments to safeguard public health.
Certified professionals, including aesthetic GPs, dermatologists and plastic surgeons, are trained in administering injectables safely and effectively. However, the use of these treatments during pregnancy and breastfeeding poses unique challenges due to the limited data available on their safety in these populations.
PREGNANCY: IMPLICATIONS FOR COSMETIC PROCEDURES
During pregnancy, a woman’s body undergoes a remarkable symphony of physiological changes, including hormonal fluctuations, alterations in blood volume and adaptations within her immune system. These biological alterations are orchestrated to safeguard both the mother and baby throughout the gestational journey. Understanding these changes is crucial in assessing the safety and efficacy of cosmetic procedures during pregnancy and breastfeeding.
HORMONAL CHANGES AND SKIN SENSITIVITY
Pregnancy hormones, including oestrogen and progesterone, exert profound effects on the skin, leading to increased sensitivity and susceptibility to conditions such as melasma and acne. These hormonal fluctuations can influence the choice of cosmetic procedures and the risk of adverse reactions.
Oestrogen, in particular, plays a key role in maintaining skin hydration and elasticity. However, elevated levels of oestrogen during pregnancy can also exacerbate certain skin conditions, such as melasma, a form of hyperpigmentation that commonly flares up or presents during pregnancy.
Progesterone, on the other hand, contributes to the development of acne by increasing sebum production and promoting inflammation within the skin’s oil glands. As a result, pregnant women may experience worsening acne during pregnancy, further complicating the selection of appropriate skincare and cosmetic interventions.
BLOOD VOLUME AND CIRCULATION ALTERATIONS
Pregnancy is characterized by significant increases in blood volume and circulation, which serve to support the developing fetus and placenta. These physiological changes can impact the absorption, distribution, and metabolism of drugs and other substances, including cosmetic injectables.
The expanded blood volume that occurs during pregnancy can lead to enhanced systemic absorption of injected substances, potentially altering their pharmacokinetic properties and increasing the risk of adverse effects. Similarly, changes in circulation can affect the distribution of injectables within the body, influencing their efficacy and safety.
IMMUNE SYSTEM ADJUSTMENTS
Pregnancy triggers a complex interplay of immune system adaptations. The immune system undergoes subtle shifts, allowing the body to tolerate the presence of the growing fetus while remaining vigilant against potential pathogens or threats. Whilst these changes are crucial for a healthy pregnancy, they also influence how the body responds to cosmetic and aesthetic procedures and the risk of complications, such as infection and delayed wound healing.
One notable adjustment involves a shift towards a more tolerant immune state, which helps prevent rejection of the developing fetus. This tolerance is orchestrated by various mechanisms, including an increase in regulatory T cells. Additionally, there is a subtle suppression of certain immune responses, particularly those that could potentially harm the fetus, such as pro-inflammatory cytokine production. Moreover, the placenta, a vital organ that facilitates nutrient and oxygen exchange between the mother and baby, plays a pivotal role in immune regulation during pregnancy. It acts as a barrier, selectively permitting the passage of substances while safeguarding against pathogens. Specialized cells within the placenta help modulate the maternal immune response, ensuring a balanced environment that supports fetal growth and development.
These immune system adjustments not only protect the developing baby but also influence how the maternal body responds to external factors, including infections, allergens, and therapeutic interventions such as cosmetic and aesthetic treatments. While the immune system is adept at maintaining this delicate equilibrium, certain procedures may pose risks during pregnancy due to the altered immune landscape. For instance, treatments involving potent medications or procedures that carry a risk of infection should be approached with caution, as they may disrupt the carefully orchestrated immune balance essential for a healthy pregnancy.
In other words: This immune tolerance can impact the body’s ability to mount an effective immune response to pathogens and foreign substances, potentially increasing the risk of infections and other complications following cosmetic procedures.
THE RISK OF AESTHETIC INJECTABLES DURING BREASTFEEDING
Breastfeeding presents unique considerations regarding the safety of aesthetic injectables, particularly with regard to potential risks to maternal health, infant safety and breast milk contamination.
Potential contamination of breast milk
Limited research suggests the possibility of systemic absorption of injectable substances and their subsequent excretion into breast milk. However, the extent to which these substances pose a risk to breastfeeding infants remains unclear, necessitating caution and thorough consultation with healthcare providers. Studies examining the presence of cosmetic injectables in breast milk have yielded conflicting results. Despite the lack of definitive data, the consensus among experts is to proceed with caution and prioritize the safety of both the mother and child.
Impact on lactation and infant health
The potential impact of cosmetic injectables on lactation and infant health is a subject of ongoing debate and research. While some studies suggest minimal systemic absorption and transmission of these substances to breastfed infants, others raised concerns about potential adverse effects on milk production and composition. Further research is needed to better understand the long-term implications of cosmetic procedures during breastfeeding.
CONSIDERATIONS DURING PREGNANCY AND BREASTFEEDING
Navigating the complex landscape of aesthetic injectables during pregnancy and breastfeeding requires careful consideration, informed decision-making, and collaboration between patients and healthcare providers.
Cosmetic procedure timing and planning
It is generally recommended to postpone elective cosmetic procedures until after pregnancy and breastfeeding to minimize potential risks to maternal health and infant safety. While some treatments may be deemed safe under certain circumstances, the lack of comprehensive safety data necessitates caution and individualized assessment.
Thorough pre-treatment consultations with healthcare providers are essential to evaluate the potential risks and benefits of aesthetic injectables in the context of pregnancy and breastfeeding. Patients should be informed of the uncertainties surrounding these treatments and empowered to make informed decisions that prioritize their health and well-being.
Alternative treatments and safe practices
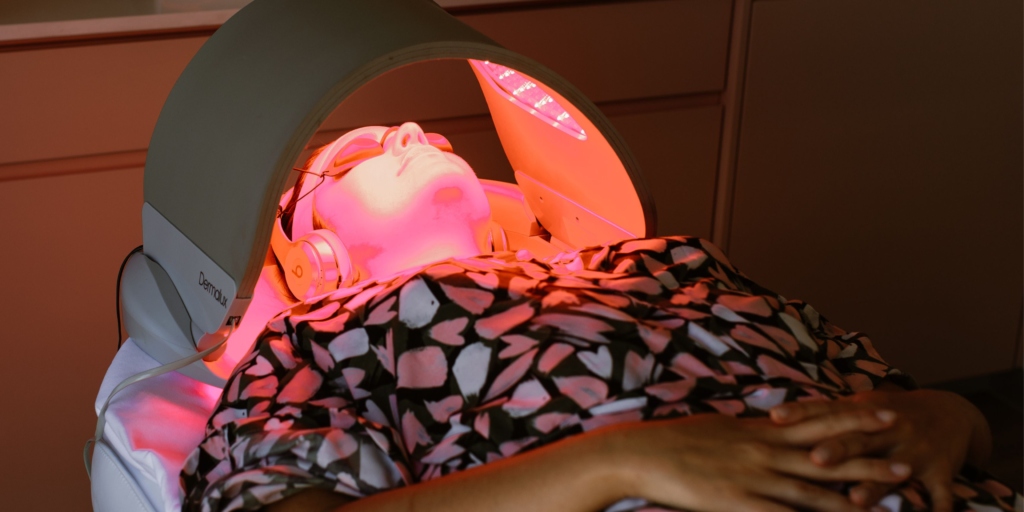
In lieu of aesthetic injectables, pregnant and breastfeeding women can explore alternative skincare treatments and practices that are considered safe and effective during these periods. Non-invasive modalities such as LED light therapy (our Dermalux LED Lights, our Bespoke Recovery Booster treatment), QMS Medicosmetics facial treatments and topical skincare products (containing scientifically studied, pregnancy-safe ingredients) offer viable alternatives for addressing common skin concerns without exposing the mother or infant to unnecessary risks.
LED light therapy, for example, harnesses the power of specific wavelengths of light to target acne-causing bacteria, reduce inflammation, and promote collagen production. This non-invasive approach is well tolerated and poses minimal risk to pregnant and breastfeeding women, making it a suitable option for managing skin conditions during these sensitive periods.
Similarly, topical skincare products formulated with gentle and pregnancy-safe ingredients, such as hyaluronic acid and antioxidants, can help maintain skin health and address common concerns like dryness, pigmentation, and fine lines. Specialized facials, chemical peels and skincare solutions tailored to the unique needs of expectant and nursing mothers are also available, providing effective yet gentle alternatives to traditional cosmetic treatments.
FINAL THOUGHTS
Whilst anecdotal reports may suggest that botulinum toxin does not reach significant enough systemic concentrations to cause adverse effects on the fetus, there are no clinical trials on the effects and safety of botulinum toxin in pregnancy. In addition, there is also a lack of evidence to support the safety of fillers during pregnancy. The prevailing consensus in the field of aesthetic injectables during pregnancy is therefore to defer treatments until after the pregnancy and breastfeeding period. By empowering women with knowledge, support, and access to safe alternatives, we can ensure that their skincare journeys are guided by wisdom, compassion, and a commitment to maternal and infant well-being.
To further explore safe skincare practices during pregnancy and breastfeeding, schedule a consultation with our team of specialists to personalize and safeguard your skincare journey during this special time.
Email us at capetown@drnwilkinson.co.za to start your pregnancy-safe Skin Science journey today.
by Aesthetic Doctor, Dr Cindy Kerbel